One-pot preparation of piperazines by regioselective ring-opening of non-activated arylaziridines†
Abstract
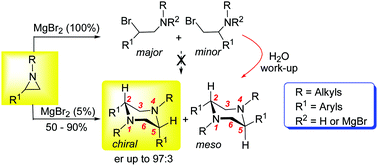
Herein we report a new straightforward synthesis of cis and trans 2,5-disubstituted N,N-dialkylpiperazines, even in enantioenriched form, by reacting non-activated N-alkyl arylaziridines in the presence of a catalytic amount of a Lewis acid. A stereochemical and NMR investigation revealed useful mechanistic insights for this process.

 Please wait while we load your content...
Please wait while we load your content...