Synthesis and antibacterial activity of novel neamine derivatives: preponderant role of the substituent position on the neamine core†
Abstract
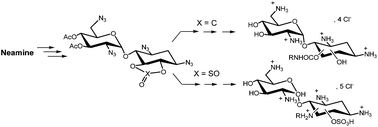
A series of neamine derivatives were prepared from the cyclic carbonate and sulfate of 1,3,2′,6′-tetraazido-3′,4′,-di-O-acetylneamine. Ring opening reactions with diversely substituted amines result in the formation of the corresponding carbamates or sulfonic acids with good overall yields. The antibacterial activities of the synthesized products against E. coli (DH5α) and S. aureus (RN4220) were evaluated. With isolated single regioisomers, the preponderant effect of the 5-positions of the carbamate substituent on the neamine core was demonstrated.

 Please wait while we load your content...
Please wait while we load your content...