Expedient synthesis of α-substituted fluoroethenes†
Abstract
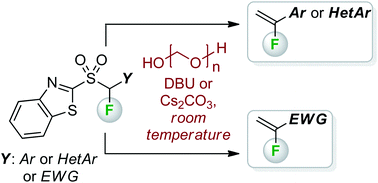
A mild and efficient synthesis of 1-aryl-1-fluoroethenes from benzothiazolyl (aryl)fluoromethyl sulfones and paraformaldehyde, under DBU- or Cs2CO3-mediated conditions at room temperature, is described. A comparable diethyl fluoro(naphthalen-2-yl)methylphosphonate reagent does not react with paraformaldehyde under these mild conditions. The utility of the methodology for synthesis of terminal α-fluoroalkenes bearing electron-withdrawing functionalities is also shown.

 Please wait while we load your content...
Please wait while we load your content...