Replication of biosynthetic reactions enables efficient synthesis of A-factor, a γ-butyrolactone autoinducer from Streptomyces griseus†
Abstract
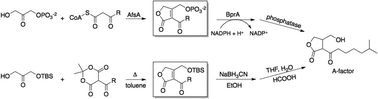
We report a concise synthesis of A-factor, the prototypical γ-butyrolactone signalling compound of Streptomyces bacteria. In analogy to enzymatic reactions in A-factor biosynthesis, our synthesis features a tandem esterification–

 Please wait while we load your content...
Please wait while we load your content...