Selectivity of arsenite interaction with zinc finger proteins†
Abstract
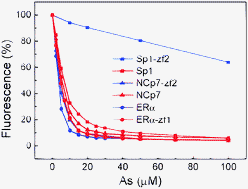
Arsenic is a carcinogenic element also used for the treatment of acute promyelocytic leukemia. The reactivity of proteins to arsenic must be associated with the various biological functions of As. Here, we investigated the selectivity of arsenite to zinc finger proteins (ZFPs) with different zinc binding motifs (C2H2, C3H, and C4). Single ZFP domain proteins were used for the direct comparison of the reactivity of different ZFPs. The binding constants and the reaction rates have been studied quantitatively. Results show that both the binding affinity and reaction rates of single-domain ZFPs follow the trend of C4 > C3H ≫ C2H2. Compared with the C2H2 motif ZFPs, the binding affinities of C3H and C4 motif ZFPs are nearly two orders of magnitude higher and the reaction rates are approximately two-fold higher. The formation of multi-domain ZFPs significantly enhances the reactivity of C2H2 type ZFPs, but has negligible effects on C3H and C4 ZFPs. Consequently, the reactivities of the three types of multi-domain ZFPs are rather similar. The 2D NMR spectra indicate that the AsIII-bound ZFPs are also unfolded, suggesting that arsenic binding interferes with the function of ZFPs.

 Please wait while we load your content...
Please wait while we load your content...