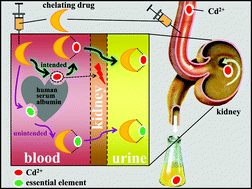
The exposure of various human populations to Cd2+ is of increasing health concern. After its gastrointestinal absorption into the bloodstream, Cd2+ binds to α2-macroglobulin and serum albumin. Although animal studies have demonstrated that meso-2,3-dimercaptosuccinic acid (DMSA) and diethylenetriamine pentaacetic acid (DTPA) can effectively mobilize Cd2+ to urine and decrease the Cd concentrations of the kidneys, the liver and the brain, not much is known about the abstraction of Cd2+ from blood plasma proteins. We prepared a stock of Cd2+ spiked rabbit plasma (2.0 μg of Cd2+/mL) and analyzed aliquots by size exclusion chromatography coupled on-line to an inductively coupled plasma atomic emission spectrometer (SEC-ICP-AES) while simultaneously monitoring the emission lines of Ca, Cd, Cu, Fe, and Zn. After the addition of 0.33 mM, 0.66 mM or 0.99 mM of DMSA, DTPA, 2,3-dimercapto-1-propanesulfonic acid (DMPS) or N-acetyl-L-cysteine (NAC) to plasma aliquots, the obtained mixtures were analyzed by SEC-ICP-AES after 5 min and 30 min. None of the investigated compounds adversely affected the plasma distribution of Fe at all investigated doses. At 0.33 mM, DTPA was most effective at mobilizing plasma protein bound Cd2+ to a ∼5 kDa Cd-species (100% removal), followed by DMPS (94%), DMSA (83%) and NAC (3%). All investigated compounds also mobilized Zn2+ from plasma proteins to ∼5 kDa Zn-species (DTPA: 80% removal; DMPS: 63%; DMSA: 29% and NAC: 3%). The addition of DTPA resulted in the dose-dependent elution of a [Ca–DTPA]3− complex. Based on these results, 0.33 mM DMSA represents the best compromise that can be achieved between maximizing the abstraction of Cd2+ from plasma proteins (83%), while minimizing the mobilization of Zn2+ from plasma proteins (29%), and avoiding the complexation of Ca2+.

 Please wait while we load your content...
Please wait while we load your content...