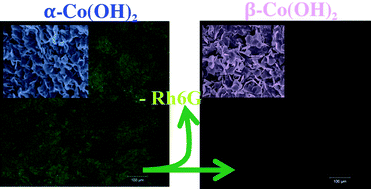
We study the kinetics and mechanism of intercalation and de-intercalation of small anions during the formation of crystalline α-Co(OH)2 and its transformation to β-Co(OH)2 within a reaction–diffusion framework. We therein use fluorescence spectroscopy with Rhodamine 6G (Rh6G) as a probe as well as other spectroscopic and imaging techniques. The method is based on the reaction and diffusion of hydroxide ions into a gel matrix containing the Co(II) ions, the conjugate anions to be intercalated and Rh6G. The advantage of this simple method is that it allows us to separate throughout space the various stages during the formation of α-Co(OH)2 and its transformation to β-Co(OH)2, thus enabling fluorescence measurements of the those stages by simply focusing on different areas of the tube. It also permits us to extract with ease the solids for characterization and image analysis. The macroscopic evolution of the system, which consists of a leading blue front designating the formation of α-Co(OH)2 followed by a sharp blue/pink interface designating the transformation to the pink β-Co(OH)2, exhibits different dynamics depending on the anion present in the gel. At a certain stage, the blue/pink interface stops its propagation and only the blue front continues. This represents clear evidence of the dependence of the kinetics of intercalation and de-intercalation on the nature of the anion. The coexisting polymorphs were collected and characterized using XRD, FTIR, Raman and UV-Vis. The fluorescence images of the α-Co(OH)2 reveal clearly the presence of Rh6G between its layers, whereas images from the β polymorph indicate the opposite. Moreover, the fluorescence of Rh6G is monitored during the formation of α-Co(OH)2 and its conversion to β-Co(OH)2. During the formation, the fluorescence intensity and lifetime are significantly increased whereas the opposite happens during the transformation to the β phase. We are able to calculate the activation energies associated with the intercalation and de-intercalation of anions and show using SEM that the polymorphic transformation is accompanied by an Ostwald ripening mechanism whereby the smaller crystals of α-Co(OH)2 dissolve to reappear as larger crystals of β-Co(OH)2. We find that the activation energies of de-intercalation are systematically smaller than those of intercalation. Our results confirm many aspects of the results of Du et al. on the conversion of a chloride-intercalated α-Co(OH)2 to β-Co(OH)2.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...