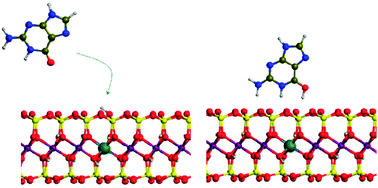
In the present study, DFT periodic plane wave calculations, at the PBE-D level of theory, were carried out to investigate the interaction of DNA nucleobases with acidic montmorillonite. The surface model was considered in its octahedral (Osub) and tetrahedral (Tsub) substituted forms, known to have different acidic properties. The adsorption of adenine, guanine and cytosine was considered in both orthogonal and coplanar orientations with the surface, interacting with the protonvia a given heteroatom. In almost all considered cases, adsorption involved the spontaneous proton transfer to the nucleobase, with a more pronounced character in the Osub structures. The binding energy is about 10 kcal mol−1 larger for Osub than for Tsub complexes mainly due to the larger acidity in Osub surfaces and due to the better stabilization by H-bond contacts between the negatively charged surface and the protonated base. The binding energy of coplanar orientations of the base is observed to be as large as the orthogonal ones due to a balance between electrostatic and dispersion contributions. Finally the binding of guanine and adenine on the acidic surface amounts to 50 kcal mol−1 while that of cytosine rises to 44 kcal mol−1.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...