(H3N–BH3)4: the ammonia borane tetramer†
Abstract
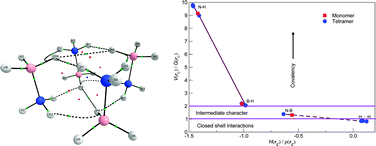
For this special edition of PCCP dealing with prediction of new molecules using quantum mechanical methods, we propose a structure for (H3N–BH3)4, the isolated ammonia borane tetramer in gas phase, for which there are no experimental reports. The structure, belonging to the S4 point group, was found at the MP2/6-311++G(d,p) level of theory; the total energy was computed at the CCSD(T) level including BSSE correction, affording a binding energy of 40.1 kcal mol−1. The tetramer is stabilized by a network of dihydrogen bonds. We study the stabilizing interactions via QTAIM, obtaining eight N–Hδ+⋯δ−H–B bonding interactions characterized as hydrogen bonds by application of the Koch–Popelier rules; in addition, two highly controversial B–Hδ−⋯δ−H–B interactions are also predicted by a topological analysis of the electron density.
- This article is part of the themed collection: Predicting new molecules by quantum chemical methods

 Please wait while we load your content...
Please wait while we load your content...