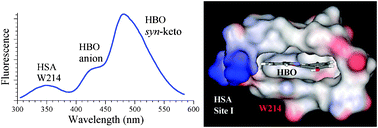
The ability of site I of human serum albumin (HSA) to bind medium sized molecules is important for the distribution, metabolism, and efficacy of many drugs. Herein, we show that this binding site has the ionization ability that may alter the drug structure during the process of its delivery. We reveal this ability by employing 2-(2′-hydroxyphenyl)benzoxazole (HBO) as a pH sensitive probe. Binding of HBO in site I is studied here at physiological pH 7.2 using steady-state and lifetime spectroscopic measurements, molecular docking and molecular dynamics (MD) simulation methods. The complex photophysics of HBO and the unique fluorescence signature of its anionic form indicate that, upon binding with HSA, the molecule exists in equilibrium between the anionic and the syn-keto forms. The position of HBO inside the binding site was determined experimentally by measuring the fluorescence quenching of W214, the sole tryptophan residue in HSA. The ionization degree of HBO inside the binding site was estimated to be close to the ionization degree of HBO in an aqueous solution of pH 10. This was concluded by comparing the fluorescence behavior of bound HBO to that of HBO in different solvents and in aqueous solutions of different pH values. Molecular docking and MD simulations show that HBO binds in site I close to W214, confirming the experimental results, and pinpoint the dominant role of hydrophobic interactions in the binding site. The formation of the anionic form is proposed to be due to through-space interaction between the OH group of HBO and both R222 and I290 with a binding mode similar to that of warfarin in site I. Comparison of the results with those of HBO mixed with key amino acids in solution indicates the importance of through-space interaction in the formation of the anion, similar to enzymatic reactions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...