Decomposition mechanism and the effects of metal additives on the kinetics of lithium alanate
Abstract
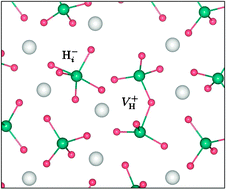
First-principles density functional theory studies have been carried out for native defects and transition-metal (Ti and Ni) impurities in lithium alanate (LiAlH4), a potential material for hydrogen storage. On the basis of our detailed analysis of the structure, energetics, and migration of lithium-, aluminium-, and hydrogen-related defects, we propose a specific atomistic mechanism for the decomposition and
 Please wait while we load your content...
Please wait while we load your content...