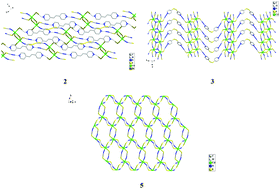
The room-temperature reactions between CdX2, NH4SCN and bidentate N-heterocyclic molecules in acidic C2H5OH/H2O solutions yielded five new thiocyanatocadmates, [H2bpp][CdCl2(SCN)2] (bpp = 1,3-bis(4-piperidyl)propane) 1, [H2bpe][CdBr2(SCN)2] (bpe = 1,2-bis(4-pyridyl)ethane) 2, [H2(4,4′-dtdpy)][Cd2(SCN)6] (4,4′-dtdpy = 4,4′-dithiodipyridine) 3, [H2(4,4′-dtdpy)][CdBr2(SCN)2] 4 and [H(2,2′-dtdpy)][Cd(SCN)3] (2,2′-dtdpy = 2,2′-dithiodipyridine) 5. It is noteworthy that (i) in compound 3, Cd2+ and SCN− aggregate together to form two types of ribbons with H2(4,4′-dtdpy)2+ as the templating agent; (ii) in compound 5, 2,2′-dtdpy is monoprotonated, and the anion [Cd(SCN)3]− shows a 2D honeycomb-like layer structure; (iii) in compounds 1–4, the N-heterocyclic molecules still act as the linkers to extend the inorganic anions into 1D or 2D supramolecular networks via weak hydrogen-bonded interactions with piperidyl/pyridyl N atoms as the acceptors, although they are diprotonated. In ethanol solution, all of the title compounds emit light upon excitation.

 Please wait while we load your content...
Please wait while we load your content...