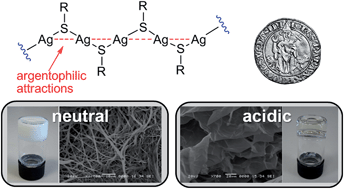
In this work we have studied the structure of silver(I) thiolate oligomers that lead to the formation of argentophilic hydrogels. The results showed two different and clearly distinguishable systems, depending on the neutral or acidic character of the thiol. Neutral systems gave opaque hydrogels, showed a fibrilar microstructure and were very dependent on the silver : thiol ratio. On the other hand, acidic hydrogels were transparent, showed a sheet-like microstructure and were not affected by the silver : thiol stoichiometry. The molecular weight of these metallophilic polymers was measured for the first time, to the best of our knowledge, by size-exclusion chromatography (SEC) and MALDI-TOF mass spectrometry. The results suggest that neutral hydrogels are composed of tetrameric species, whereas the acidic ones are composed of slightly longer oligomers (n = 8–14). In addition, several observations have shown the dynamic character of these oligomeric systems, opening the way for a novel and facile approach towards the fabrication of new multiresponsive soft materials.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...