pH and temperature dependent relaxation dynamics of Hoechst-33258: a time resolved fluorescence study
Abstract
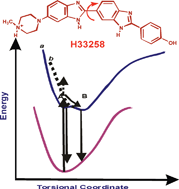
The photophysical behavior of Hoechst 33258 (H33258) in aqueous solution has been studied by steady-state and time-resolved fluorescence measurements. The intriguing intramolecular geometrical orientations of the

 Please wait while we load your content...
Please wait while we load your content...