Photocatalytic discoloration of aqueous malachite green solutions by UV-illuminated TiO2nanoparticles under air and nitrogen atmospheres: effects of counter-ions and pH
Abstract
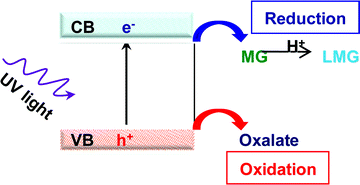
Under air atmosphere, the photocatalytic discoloration of
* Corresponding authors
a
Centro de Investigación y Desarrollo en Ciencias Aplicadas “Dr J. J. Ronco” (CINDECA), Departamento de Química, Facultad de Ciencias Exactas, UNLP-CCT La Plata, CONICET, 47 No. 257, 1900 La Plata, Buenos Aires, Argentina
E-mail:
julianregifo@quimica.unlp.edu.ar
Fax: +54 221 4211353 ext.125
Tel: +54 221 421353
b SB-ISIC-GGEC, Station 6, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
c SB-SCGC, Station 6, Ecole Polytechnique Fédéral de Lausanne, Lausanne, Switzerland
d SB-ISIC, Station 6, Ecole Polytechnique Fédéral de Lausanne, Lausanne, Switzerland
Under air atmosphere, the photocatalytic discoloration of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
J. A. Rengifo-Herrera, L. R. Pizzio, M. N. Blanco, C. Roussel and C. Pulgarin, Photochem. Photobiol. Sci., 2011, 10, 29 DOI: 10.1039/C0PP00196A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
