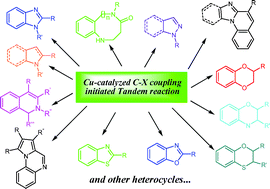
Tandem reactions initiated by copper-catalyzed cross-coupling: A new strategy towards heterocycle synthesis
Abstract
Copper-catalyzed cross-coupling reactions which lead to the formation of C–N, C–O, C–S and C–C bonds have been recognized as one of the most useful strategies in synthetic organic chemistry. During past decades, important breakthroughs in the study of Cu-catalyzed coupling processes demonstrated that Cu-catalyzed reactions are broadly applicable to a variety of research fields related to organic synthesis. Representatively, employing these coupling transformations as key steps, a large number of tandem reactions have been developed for the construction of various

 Please wait while we load your content...
Please wait while we load your content...