Enantioselective synthesis of pyranonaphthoquinone antibiotics using a CBS reduction/cross-metathesis/oxa-Michael strategy†
Abstract
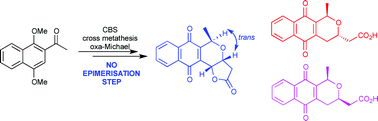
The enantioselective syntheses of deoxydihydrokalafungin (5), cis-deoxydihydrokalafungin (6) and deoxykalafungin (7) are reported. The strategy was based on 4 key reactions: (1) CBS reduction of prochiral ketone 10 to introduce chirality at C-1, (2) radical allylation of quinone 9a, (3) cross-metathesis of dimethoxynaphthalene 13 with methyl acrylate, and (4) intramolecular oxa-Michael addition of alcohol 8 to form the core naphthopyran ring system. This novel approach delivers naphthopyrans possessing the natural trans-stereochemistry observed in the pyranonaphthoquinone family of antibiotics.

 Please wait while we load your content...
Please wait while we load your content...