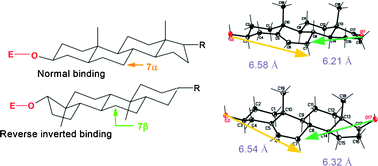
Hydroxylation of DHEA, androstenediol and epiandrosterone by Mortierella isabellinaAM212. Evidence indicating that both constitutive and inducible hydroxylases catalyze 7α- as well as 7β-hydroxylations of 5-ene substrates
Abstract
The course of transformation of DHEA,

 Please wait while we load your content...
Please wait while we load your content...