Pyrroloquinoxalinehydrazones as fluorescent probes for amyloid fibrils†
Abstract
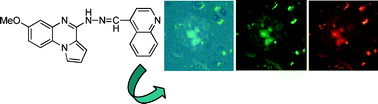
Here we describe the identification and preliminary characterization of a new class of pyrrolo(imidazo)quinoxaline
* Corresponding authors
a
European Research Centre for Drug Discovery and Development (NatSynDrugs), University of Siena, via Aldo Moro, Siena, Italy
E-mail:
campiani@unisi.it
Fax: 39 0577 234333
Tel: 39 0577 234172
b
Dip. Farmaco Chimico Tecnologica, University of Siena, via Aldo Moro, Siena, Italy
E-mail:
campiani@unisi.it
Fax: 39 0577 234333
Tel: 39 0577 234172
c Istituto di Ricerche Farmacologiche “Mario Negri” (IRFMN), Via la Masa 19, Milano, Italy
d Dip. di Chimica, University of Siena, via Aldo Moro, Siena, Italy
e Dip. di Chimica delle Sostanze Naturali, Università di Napoli Federico II, via D. Montesano 49, Napoli, Italy
f Dip. di Chimica Farmaceutica e Tossicologica, Università di Napoli Federico II, via D. Montesano 49, Napoli, Italy
Here we describe the identification and preliminary characterization of a new class of pyrrolo(imidazo)quinoxaline

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
S. Gemma, L. Colombo, G. Forloni, L. Savini, C. Fracasso, S. Caccia, M. Salmona, M. Brindisi, B. P. Joshi, P. Tripaldi, G. Giorgi, O. Taglialatela-Scafati, E. Novellino, I. Fiorini, G. Campiani and S. Butini, Org. Biomol. Chem., 2011, 9, 5137 DOI: 10.1039/C1OB05288H
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
