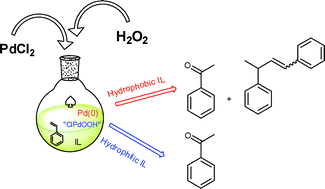
A series of hydrophilic N,N-dimethylpyrrolidinium- and N,N-dimethylpiperidinium-based ionic liquids (ILs) have been prepared and applied as reaction media in the Wacker oxidation of styrene by hydrogen peroxide using PdCl2 as the catalyst. The efficiency of these ILs was compared with hydrophilic and hydrophobic imidazolium systems (including those with nitrile functionalities). The nature of the ionic liquid strongly influences the product distribution. In particular, in hydrophobic ILs, relevant amounts of 1,3-diphenyl-1-butene arising from styrene dimerization were detected, in addition to the expected phenylmethylketone. The formation of 1,3-diphenyl-1-butene may be attributed to the formation of Pd(0) species from “ClPdOH” (probably formed during the Wacker process) in a side-reaction. Consequently, the ability of the IL to favor or disfavor the reoxidation of “ClPdOH” to “ClPdOOH” by hydrogen peroxide, giving an homogeneous phase or a biphasic system, appears to be the main factor affecting selectivity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...