The reactions of the 16e half-sandwich complex (p-cymene)Ru(S2C2B10H10) (Ru16e) with 1,4-diethynylbenzene (L1), 3′,6-diethynyl-1,1′-binaphthyl-2,7′-diyl diacetate (L2), 2-bromo-5-ethynylthiophene (L3) and 2,5-diethynylthiophene (L4) lead to 18e mononuclear complexes (p-cymene)Ru(S2C2B10H9)(H2CCPhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) (1), (p-cymene)Ru(S2C2B10H9)[H2CC(C24H16O4)C
CH) (1), (p-cymene)Ru(S2C2B10H9)[H2CC(C24H16O4)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH] (2), (p-cymene)Ru(S2C2B10H9) [H2CC(C4H2S)Br] (3) and (p-cymene)Ru(S2C2B10H9) [H2CC(C4H2S)C
CH] (2), (p-cymene)Ru(S2C2B10H9) [H2CC(C4H2S)Br] (3) and (p-cymene)Ru(S2C2B10H9) [H2CC(C4H2S)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH] (4), respectively. In all of them, metal-induced B–H activation has occurred, which leads to a stable Ru–B bond, and the structures take a cisoid arrangement. Only in the case of L4, the binuclear complexes [(p-cymene)Ru(S2C2B10H9)]2[H2CC(C4H2S)CCH2] (5a and 5b) are observed, which are conformational isomers generated by the differing orientations of the p-cymene unit. 4 can be readily converted to the complex (p-cymene)Ru(S2C2B10H9)[H2CC(C4H2S)COCH3] (6) in the presence of silica and H2O. All of these products 1–6 were characterized by NMR, IR, elemental analysis and mass spectrometry. The structures of 1, 3, and 5a were also determined by single-crystal X-ray diffraction analysis.
CH] (4), respectively. In all of them, metal-induced B–H activation has occurred, which leads to a stable Ru–B bond, and the structures take a cisoid arrangement. Only in the case of L4, the binuclear complexes [(p-cymene)Ru(S2C2B10H9)]2[H2CC(C4H2S)CCH2] (5a and 5b) are observed, which are conformational isomers generated by the differing orientations of the p-cymene unit. 4 can be readily converted to the complex (p-cymene)Ru(S2C2B10H9)[H2CC(C4H2S)COCH3] (6) in the presence of silica and H2O. All of these products 1–6 were characterized by NMR, IR, elemental analysis and mass spectrometry. The structures of 1, 3, and 5a were also determined by single-crystal X-ray diffraction analysis.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) (1), (p-cymene)Ru(S2C2B10H9)[H2CC(C24H16O4)C
CH) (1), (p-cymene)Ru(S2C2B10H9)[H2CC(C24H16O4)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH] (2), (p-cymene)Ru(S2C2B10H9) [H2CC(C4H2S)Br] (3) and (p-cymene)Ru(S2C2B10H9) [H2CC(C4H2S)C
CH] (2), (p-cymene)Ru(S2C2B10H9) [H2CC(C4H2S)Br] (3) and (p-cymene)Ru(S2C2B10H9) [H2CC(C4H2S)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH] (4), respectively. In all of them, metal-induced B–H
CH] (4), respectively. In all of them, metal-induced B–H 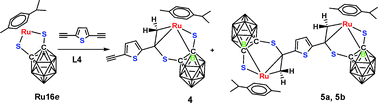

 Please wait while we load your content...
Please wait while we load your content...