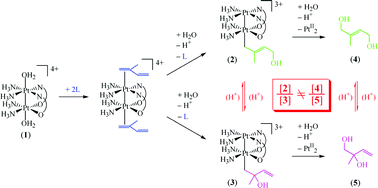
Reactions of a pivalamidato-bridged head-to-head (HH) platinum(III) binuclear complex with 2-methyl-1,3-butadiene (isoprene) and p-styrenesulfonate and of an α-pyrrolidonato-bridged HH platinum(III) binuclear complex with p-styrenesulfonate were studied kinetically using UV-vis spectrophotometry and 1H NMR spectroscopy, and detailed reaction mechanisms are proposed. Pt(III) binuclear complexes react with p-styrenesulfonate in four successive steps with mechanisms similar to that for an HH α-pyridonato-bridged Pt(III) binuclear complex with p-styrenesulfonate. In the case of isoprene, four steps were observed on the basis of UV-vis spectrophotometry. However, the reaction kinetics for steps 1 and 2 correspond to those for the previous reaction system, and those for steps 3 and 4 do not correspond to those for the previous system or to those observed by using 1H NMR spectroscopy for the present isoprene system. By using UV-vis spectrophotometry, it was shown that isoprene preferentially π-coordinates to the Pt(N2O2) atom via the double bond adjacent to the methyl group in step 1. In step 2, a second isoprene molecule π-coordinates to the Pt(N4) atom, which is the rate-determining step, followed by nucleophilic attack of a water molecule on the π-coordinated isoprene on the Pt(N2O2) atom to form two isomeric σ-complexes. In the same step, π-coordinated isoprene on the Pt(N4) atom of the σ-complexes is released. This is different from the reaction of the Pt(III) binuclear complexes with other olefins. In step 3, reductive elimination of the σ-complexes occurs to form two diols and the HH pivalamidato-bridged Pt(II) binuclear complex. Finally, acid decomposition of the Pt(II) binuclear complex occurs to form monomers in step 4. From 1H NMR spectroscopic observations, fast isomerization between σ-complexes and reductive elimination of the σ-complexes occurs in step 3, and isomerization from a 1,4-diol to a 1,2-diol occurs in step 4.

 Please wait while we load your content...
Please wait while we load your content...