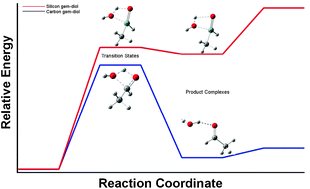
Activation barrier heights for the dehydration reaction of geminal carbinols and silanediols R′R″X(OH)2 (X = C, Si) were estimated at the B3LYP and MP2 levels of theory employing Dunning's correlation-consistent triple-zeta basis sets. It was shown that the barrier height for carbon derivatives steadily decreases upon substitution by R groups, usually termed as electron-donating, such as alkyl and amino groups. Substitution by electron-withdrawing groups leads, however, only to small changes in barrier heights compared to that of methanediol. A similar tendency was also found for silicon derivatives, but high activation barriers of this reaction remain even for amino group substituted silanediols. Introduction of additional water molecules into the reactive space of carbinol dehydration drastically reduces barrier heights and brings the transition state energy for methanediol close to the experimental value. The difference between dehydration barrier heights for both methanediol and carbinols with electron-rich substituents becomes well-defined for dimeric species. The higher acidity of the hydroxyl group protons in molecules containing halogens and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups brings about a noticeable growth in the dehydration barrier heights of these compounds. This difference in barrier heights for oligomeric species may be the reason for the stability of carbinols with electron-rich substituents.
O groups brings about a noticeable growth in the dehydration barrier heights of these compounds. This difference in barrier heights for oligomeric species may be the reason for the stability of carbinols with electron-rich substituents.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups brings about a noticeable growth in the dehydration barrier heights of these compounds. This difference in barrier heights for oligomeric species may be the reason for the stability of carbinols with electron-rich substituents.
O groups brings about a noticeable growth in the dehydration barrier heights of these compounds. This difference in barrier heights for oligomeric species may be the reason for the stability of carbinols with electron-rich substituents.

 Please wait while we load your content...
Please wait while we load your content...