Nature of adhesion of condensed organic films on platinum by first-principles simulations†
Abstract
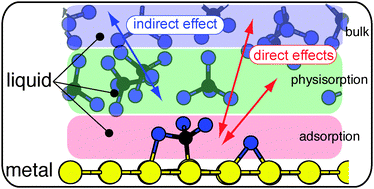
Understanding the nature of the adhesion of an organic liquid on a metal surface is of paramount importance for elucidating the stability and chemical reactivity at these complex interfaces. However, to date, the morphology, layering and chemical properties at organic liquid metal interfaces have been rarely known. Using semi-empirical dispersion corrected density functional theory calculations and ab initio molecular dynamics simulations, we show that carbon tetrachloride and

 Please wait while we load your content...
Please wait while we load your content...