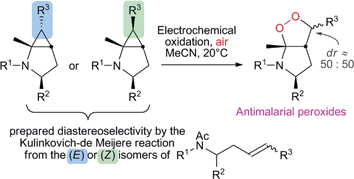
This contribution describes the synthesis of several novel bicyclic α-amino endoperoxides, including CF3-substituted compounds, prepared by the aerobic electrochemical oxidation of a family of bicyclic aminocyclopropanes. These, in turn, are readily synthesised by a titanium-mediated intramolecular cyclopropanation process (Kulinkovich–de Meijere reaction), starting from N-alkenyl amides that contain a vic-disubstituted double bond, with high diastereoselectivity. An evaluation of the biological activities of several of the molecules produced, against the parasite Plasmodium falciparum, is also presented.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...