The reactions of three isomers of phenyl-substituted propene, trans-β-methylstyrene, α-methylstyrene, and allylbenzene, with atomic oxygen adsorbed on Au(111) have been investigated in order to probe the effect of molecular structure on the selectivity for competing oxidation pathways. Temperature programmed reaction experiments show that these three isomers share common features in their reaction patterns, providing direct observation that both allylic C–H activation and epoxidation are important reaction pathways that compete with each other. The pathways observed for reaction are (1) oxygen addition to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond to yield the respective epoxides; (2) allylic C–H activation including oxygen insertion into C–H bonds to form ketone, aldehyde, or acid and C–H dissociation to initiate combustion and carbon deposition; and, (3) nucleophilic attack of the electron-deficient carbon that is adjacent to the phenyl ring to produce benzoic acid. The selectivity for epoxidation is different for the three isomers, reflecting the intrinsic gas-phase acidity of their allylic protons. Specifically, trans-β-methylstyrene (ΔGacid 401.6 kcal mol−1) and α-methylstyrene (ΔGacid 405.5 kcal mol−1), which exhibit similar gas-phase acidity determined by density functional theory (DFT) calculations, both produce epoxide. In contrast, there is no detectable epoxidation of allylbenzene, which is computed to have the highest acidity (ΔGacid 356.0 kcal mol−1), ∼ 50 kcal mol−1 relative to the other two isomers. These results provide new insight into the catalytic oxidation of allylic olefins that, in conjunction with their adsorption geometry on the surface, the gas-phase acidity also plays an essential role in determining the reaction patterns.
C bond to yield the respective epoxides; (2) allylic C–H activation including oxygen insertion into C–H bonds to form ketone, aldehyde, or acid and C–H dissociation to initiate combustion and carbon deposition; and, (3) nucleophilic attack of the electron-deficient carbon that is adjacent to the phenyl ring to produce benzoic acid. The selectivity for epoxidation is different for the three isomers, reflecting the intrinsic gas-phase acidity of their allylic protons. Specifically, trans-β-methylstyrene (ΔGacid 401.6 kcal mol−1) and α-methylstyrene (ΔGacid 405.5 kcal mol−1), which exhibit similar gas-phase acidity determined by density functional theory (DFT) calculations, both produce epoxide. In contrast, there is no detectable epoxidation of allylbenzene, which is computed to have the highest acidity (ΔGacid 356.0 kcal mol−1), ∼ 50 kcal mol−1 relative to the other two isomers. These results provide new insight into the catalytic oxidation of allylic olefins that, in conjunction with their adsorption geometry on the surface, the gas-phase acidity also plays an essential role in determining the reaction patterns.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond to yield the respective epoxides; (2) allylic C–H
C bond to yield the respective epoxides; (2) allylic C–H 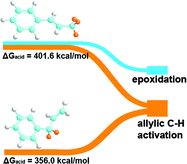

 Please wait while we load your content...
Please wait while we load your content...