Determination of relative tensor orientations by γ-encoded chemical shift anisotropy/heteronuclear dipolar coupling 3D NMR spectroscopy in biological solids†
Abstract
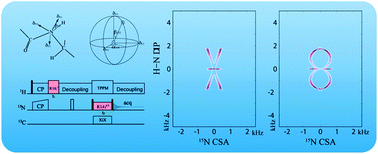
In this paper, we present 3D chemical shift anisotropy (CSA)/dipolar coupling correlation experiments, based on γ-encoded R-type symmetry sequences. The γ-encoded correlation

 Please wait while we load your content...
Please wait while we load your content...