Breaking the passivation—the road to a solvent free borohydride synthesis
Abstract
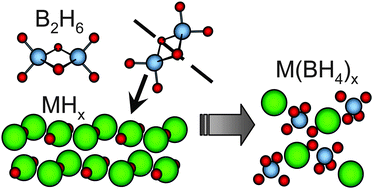
We describe a new method for the solvent-free synthesis of borohydrides at room temperature and demonstrate its feasibility by the synthesis of three of the most discussed borohydrides at present: LiBH4, Mg(BH4)2 and Ca(BH4)2. This new gas–solid mechanochemical

 Please wait while we load your content...
Please wait while we load your content...