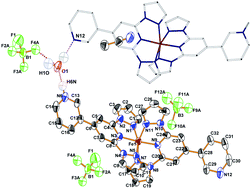
We report on the synthesis of spin transition compounds 1, 2 of formula [Fe(L)2](A)2 (where L = 2′,6′-bis(pyrazol-1-yl)-3,4′-bipyridine, A = ClO4−—compound 1; A = BF4−—compound 2) and compound 3 of formula [Fe(L)(LH)](BF4)3·H2O·CH3CN (where LH = 3-(2,6-bis(pyrazol-1-yl) pyridine-4-yl)-pyridinium(+)). Compounds 1, 2 and 3 were characterized by single-crystal X-ray diffraction, ESI-ToF mass spectrometry, 1H NMR and elemental analysis. The single-crystal X-ray diffraction study of the counter anion analogues 1 and 2 reveals almost identical molecular structures without any significant presence of intermolecular interactions. However, in the case of compound 3, the crystal structure reveals supramolecular interactions involving molecular cations, BF4− anions and, most importantly, lattice solvent molecules. The presence of solvent water molecules induces the presence of two different types of hydrogen bonding: (i) water molecules interacting with the fluorine atoms of BF4− anions and (ii) water molecules interconnecting protonated and nonprotonated nitrogens of pyridine-3-yl substituents of neighboring complex cations. These overall hydrogen bonding pattern between the neighboring iron(II) complex cation moieties is responsible for the formation of a one dimensional (1D) hydrogen bonded zig-zag chain. The magnetic investigations elucidate high temperature spin transition behavior for both anion analogues 1 and 2, while compound 3 exhibits a lattice-solvent dependency of the temperature-driven spin transition accompanied with stepwise solvent liberation above room temperature. After complete solvent removal the solvent-free compound 3d, [Fe(L)(LH)](BF4)3, shows an abrupt spin transition accompanied with thermal hysteresis loop; T1/2(↑) = 240 K and T1/2(↓) = 231 K, ΔT1/2 = 9 K. The Ising-like model that includes two vibrational modes has been applied in a direct fitting of magnetic data. The model recovers the temperature evolution of the χT product functions for all compounds under study, involving also compound 3d with the thermal hysteresis.

 Please wait while we load your content...
Please wait while we load your content...