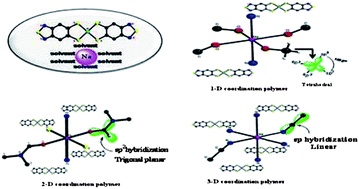
Dimensionality of coordination polymers decided by the type of hybridization of the central carbon atom of the solvent molecule that coordinates to an alkali metal cation: from discrete to 3D networks based on a gold(iii) bis(dithiolene) complex†
Abstract
The

 Please wait while we load your content...
Please wait while we load your content...