A site selective C–H arylation of free-(NH2) adenines with aryl chlorides: Application to the synthesis of 6,8-disubstituted adenines†
Abstract
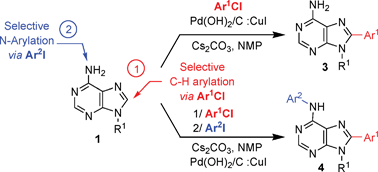
An efficient site selective method for the direct arylation of free-(NH2) adenines 1 to provide a range of C-8 arylated adenines 3 in excellent yields is described. This process based on the use of Pd(OH)2/C as the

 Please wait while we load your content...
Please wait while we load your content...