Synthesis of (±)-likonide B (smenochromene D) using a regioselective Claisen rearrangement, separation of the enantiomers and stereochemical assignment†
Abstract
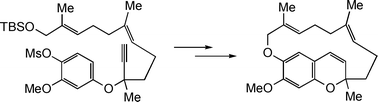
A synthesis of the unusual ansa-bridged farnesyl hydroquinone derivative likonide B in racemic form is described. The natural product, also known as smenochromene D, was obtained from

 Please wait while we load your content...
Please wait while we load your content...