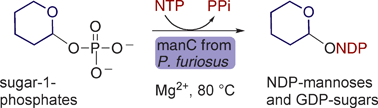
Herein we present an analysis of the chemical function of a recombinant bifunctional phosphomannose isomerase/GDP-mannose pyrophosphorylase (manC) from Pyrococcus furiosusDSM 3638 and its use in the synthesis of guanidinediphospho-hexoses and a range of nucleotidediphospho-mannoses. This enzyme is unusually promiscuous in both its nucleotide triphosphate (NTP) and sugar-1-phosphate acceptance. It accepts all five naturally occurring NTPs (ATP, CTP, GTP, dTTP and UTP) and a range of sugar-1-phosphates (glucose-, mannose-, galactose-, glucosamine-, N-acetylglucosamine- and fucose-1-phosphate). A truncated GDP-mannose pyrophosphorylase domain of the whole length enzyme showed almost 100-fold less sugar nucleotidyltransferase activity with only GTP and mannose 1-phosphate as substrates. The temperature stability and inherently broad substrate tolerance of this archaeal enzyme make it an effective reagent for the rapid chemoenzymatic synthesis of a range of natural and unnatural sugar nucleotides that are challenging to make by chemical means alone.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...