Red fluorescence from tautomers of 2′-hydroxychalcones induced by intramolecular hydrogen atom transfer
Abstract
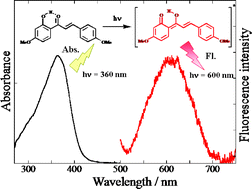
Tautomer fluorescence in the longer wavelength region at 600 nm produced by intramolecular
- This article is part of the themed collection: New Horizons of Photochromism

 Please wait while we load your content...
Please wait while we load your content...