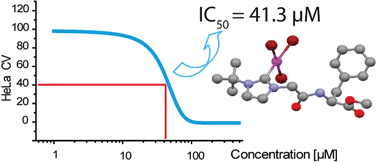
We report the synthesis of new NHC gold(I) and NHC gold(III) halide, amino acid and dipeptide complexes. Transmetallation of the N-phenylalanine-substituted NHC silver complex 3 with Me2SAuCl yields the phenylalanine–NHC gold(I) conjugate 4a. Halide exchange with LiBr and oxidation of 4a with Br2 in CH2Cl2 yields the phenylalanine–NHC Au(I) and Au(III) bromides 4b and 4c, respectively. Reaction of N-Boc protected cysteine methyl ester (Boc–Cys–OMe) or the dipeptideN-Boc–Leu–Cys–OMe with the NHC gold chloride 6a yields the (NHC)Au–S complexed amino acid and dipeptide derivatives 8 and 9. The NHC gold(III) complexes 4c and 6c were characterised by single crystal X-ray analysis. All of the tested gold carbene complexes showed significant anti-tumor activity on the HeLa, HepG2 and HT-29 cancer cell lines. The best compounds show activity comparable to the well-known anti-cancer drug cisplatin. There seems to be no clear cut structure–activity relationship in the compounds tested, nor did we observe a dependence on the metal oxidation state or the different halide substituents. Given the ease of preparation, stability and high activity of the compounds described herein, it may be possible to design tumor-specific anti-cancer agents based on NHC gold amino acid conjugates in the future.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...