Synthesis, structure, magnetic properties and DNA cleavage of binuclear Cu(ii) Schiff-base complexes†
Abstract
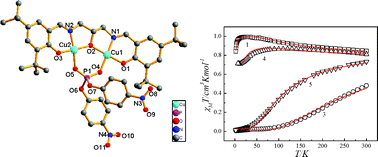
Five binuclear Schiff base copper(II) complexes [Cu2(L)(OAc)]·3DMF (1), [Cu2(L)(OAc)]2·3DMF (2), [Cu2(L)(BNPP)]·3CH3CN (3), [Cu2(L)(Fa)]·2DMF (4) and [Cu2(L)(Pa)]·DMF (5) (H3L = N,N′-bis(3,5-tert-butylsalicylidene-2-hydroxy)-1,3-propanediamine, OAc = acetic acid, BNPP = bis(4-nitrophenyl)phosphate, Fa = 2-tetrahydrofuroic acid, Pa = benzoic acid) have been synthesized and characterized by X-ray single-crystal structure analysis. Variable-temperature magnetic susceptibility studies (2–300 K) indicate the existence of ferromagnetic coupling between the copper(II) ions in complexes 1 and 4, and antiferromagnetic coupling in complexes 3 and 5. The interaction of these complexes with CT-DNA has been studied by using absorption and emission spectral methods. The apparent binding constant (Kapp) values for complexes 1, 3, 4 and 5 are 4.67 × 105, 9.48 × 105, 4.30 × 105 and 3.90 × 105 M−1, respectively, which show that the complexes bind to DNA by moderate intercalative binding modes. Furthermore, all these complexes can cleave plasmid DNA to nicked DNA in a sequential manner as the concentrations or reaction times are increased in the absence of reducing agent. Their cleavage activities are promoted in the presence of hydrogen peroxide. The cleavage mechanisms between the complexes and plasmid DNA are likely to involve singlet oxygen 1O2 and ˙OH as reactive oxygen species.

 Please wait while we load your content...
Please wait while we load your content...