Silica-bound copper(ii)triazacyclononane as a phosphate esterase: effect of linker length and surface hydrophobicity
Abstract
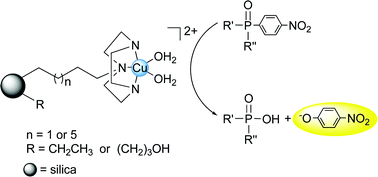
A series of silica-bound Cu(II) triazacyclononane materials was prepared to study the effect of linker length and surface hydrophobicity on the hydrolysis of

 Please wait while we load your content...
Please wait while we load your content...