Synthesis and structures of titanium and yttrium complexes with N,N′-tetramethylguanidinate ligands: different reactivity of the M–N bonds toward phenyl isocyanate†
Abstract
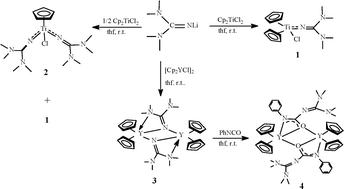
A salt ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NMe2)2] proceeded in THF at room temperature to yield a bis(cyclopentadienyl)titanium mono-guanidinate chloride (C5H5)2TiCl(N
C(NMe2)2] proceeded in THF at room temperature to yield a bis(cyclopentadienyl)titanium mono-guanidinate chloride (C5H5)2TiCl(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NMe2)2) (1). However, treatment of two equiv. of LiN
C(NMe2)2) (1). However, treatment of two equiv. of LiN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NMe2)2 with (C5H5)2TiCl2 under the same conditions resulted in the elimination of one
C(NMe2)2 with (C5H5)2TiCl2 under the same conditions resulted in the elimination of one ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NMe2)2]2 (2), in which only one Ti–Cl bond is broken, with the other Ti–Cl bond retained. Reaction of [(C5H5)2YCl]2 with LiN
C(NMe2)2]2 (2), in which only one Ti–Cl bond is broken, with the other Ti–Cl bond retained. Reaction of [(C5H5)2YCl]2 with LiN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NMe2)2 gave the corresponding product {(C5H5)2Y[μ-η1:η2-N
C(NMe2)2 gave the corresponding product {(C5H5)2Y[μ-η1:η2-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NMe2)2]}2 (3). On further investigations on the reactivity of 1–3 toward
C(NMe2)2]}2 (3). On further investigations on the reactivity of 1–3 toward ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NMe2)2)NPh)]2 (4). Complexes 1–4 were characterized by
C(NMe2)2)NPh)]2 (4). Complexes 1–4 were characterized by

 Please wait while we load your content...
Please wait while we load your content...