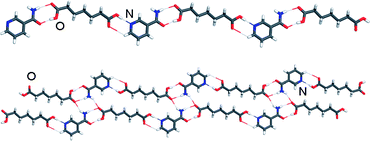
The formation of stoichiometric variations, i.e. cocrystals composed of identical molecular building blocks in different stoichiometric ratios, has been investigated using cocrystals composed of the model pharmaceutical component nicotinamide (na) and 10 dicarboxylic acids as cocrystal formers. The comparison of cocrystallisation from solution, from the melt and by neat and liquid-assisted grinding revealed that the mechanochemical methods are more efficient in screening for stoichiometric variations of cocrystals. Using grinding, for example, the formation of stoichiometric variations could be readily controlled by modifying the composition of the reaction mixture. The ability of different stoichiometric variations to interconvert using liquid-assisted grinding was also investigated, providing a tentative qualitative assessment of the relative stabilities of different cocrystal compositions. Grinding for short periods was utilised to investigate the mechanism of formation for the hydrogen-bonded cocrystals of na and suberic acid (sub). The results suggest that the cocrystal formation occurs in a stepwise manner, wherein the cocrystal (na)·(sub) appears as an intermediate in the synthesis of the (na)2·(sub) cocrystal, most likely for kinetic reasons.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...