Highly enantioselective intramolecular aza-spiroannulation onto indoles using chiral rhodium catalysis: asymmetric entry to the spiro-β-lactam core of chartellines†
Abstract
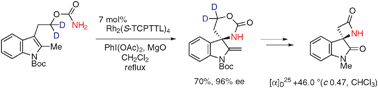
A versatile, highly enantiocontrolled entry to the spiro-β-lactam core of chartellines has been developed by expanding the scope of oxidative

 Please wait while we load your content...
Please wait while we load your content...