Synthesis of zinc bacteriochlorophyll-d analogues with various 17-substituents and their chlorosomal self-aggregates in non-polar organic solvents†‡
Abstract
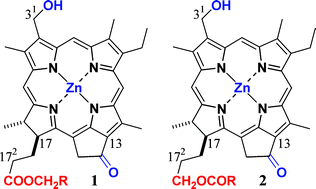
Zinc 31-demethyl-bacteriopheophorbides-d possessing various 17-propionate residue (172-COOCH2–) were prepared as models of light-harvesting pigments in major photosynthetic antennae of green bacteria. The synthetic compounds were monomeric in polar organic
- This article is part of the themed collection: Tetrapyrroles

 Please wait while we load your content...
Please wait while we load your content...