Covalently linked zinc chlorophyll dimers as a model of a chlorophyllous pair in photosynthetic reaction centers†‡
Abstract
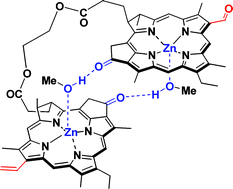
A heterodimer, where zinc pyropheophorbide-a was linked with zinc pyropheophorbide-d through ethylene glycol ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C131 bonding. Such complexes had furthest red (Qy) absorption bands at longer wavelengths than the monomeric species. These red-shifts were ascribable to excitonic coupling of the Qy transition states in the
C131 bonding. Such complexes had furthest red (Qy) absorption bands at longer wavelengths than the monomeric species. These red-shifts were ascribable to excitonic coupling of the Qy transition states in the
- This article is part of the themed collection: Tetrapyrroles

 Please wait while we load your content...
Please wait while we load your content...