Catalytic asymmetric synthesis of the alkaloid (+)-myrtine†
Abstract
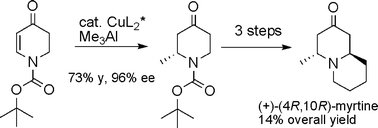
A new protocol for the asymmetric synthesis of trans-2,6-disubstituted-4-piperidones has been developed using a catalytic enantioselective conjugate addition reaction in combination with a diastereoselective lithiation–substitution sequence; an efficient synthesis of (+)-myrtine has been achieved via this route.

 Please wait while we load your content...
Please wait while we load your content...