A facile Zr-mediated multicomponent approach to arylated allylic alcohols and its application to the synthesis of highly substituted indenes and spiroindenes†
Abstract
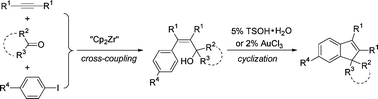
An efficient and one-pot procedure for the synthesis of arylated and stereodefined allylic

 Please wait while we load your content...
Please wait while we load your content...