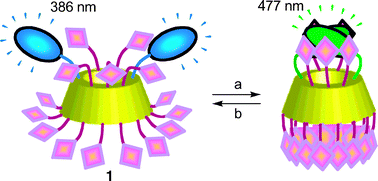
A bis(pyrene)-functionalized β-cyclodextrin (1) has been prepared in two steps from perbenzylated β-cyclodextrin. This compound shows dual emission properties, which arise from the pyrenyl chromophores. Upon excitation of 1 at 355 nm, monomer blue fluorescence (386, 407 and 428 nm) is observed in DMSO solution, whereas excimer green fluorescence (477 nm) is seen upon addition of ≥20 vol% water in DMSO. This suggests that modified β-cyclodextrin 1 changes its shape in response to the environment. The sensing properties of 1 towards carboxylic acids and alcohols were investigated in H2O–DMSO (80 : 20 v/v). Monomer fluorescence is restored selectively by medium length normal carboxylic acids, such as enanthic acid (C7) found in rancid oils and capric acid (C10), while both monomer and excimer emissions are enhanced by homologous alcohols, such as 1-heptanol, used in cosmetics and 1-decanol. Remarkably, bulkier substrates, such as tert-butyl alcohol or 1-adamantylcarboxylic acid are not detected.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...