Microcirculation within grooved substrates regulates cell positioning and cell docking inside microfluidic channels†
Abstract
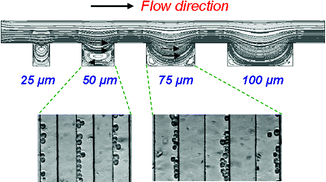
Immobilization of cells inside microfluidic devices is a promising approach for enabling studies related to
* Corresponding authors
a
Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, 65 Landsdowne Street, Room 252, Cambridge, MA, USA
E-mail:
alik@mit.edu
Fax: +1 617-768-8477
Tel: +1 617-768-8395
b Center for Biomedical Engineering, Department of Medicine, Brigham and Womens’ Hospital, Harvard Medical School, MA, USA
c Laboratory of Biological Structure Mechanics, Department of Structural Engineering, Politecnico di Milano, Milano, Italy
d IRCCS Istituto Ortopedico Galeazzi, Milano, Italy
e Division of Biopharmacy and Pharmacokinetics and Drug Discovery and Development Technology Center, University of Helsinki, Finland
Immobilization of cells inside microfluidic devices is a promising approach for enabling studies related to

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
A. Manbachi, S. Shrivastava, M. Cioffi, B. G. Chung, M. Moretti, U. Demirci, M. Yliperttula and A. Khademhosseini, Lab Chip, 2008, 8, 747 DOI: 10.1039/B718212K
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
