Mechanistic implications of the active species involved in the oxidation of hydrocarbons by iron complexes of pyrazine-2-carboxylic acid†
Abstract
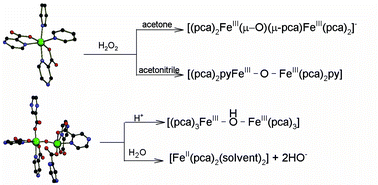
The reactivity towards H2O2 of the complexes [Fe(pca)2(py)2]·py (1) and Na2{[Fe(pca3)]2O}·2H2O·CH3CN (2) (where pca− is

 Please wait while we load your content...
Please wait while we load your content...