Electrical conductivity and oxygen nonstoichiometry of acceptor (Ga)-doped titania
Abstract
Electrical conductivity and oxygen nonstoichiometry (δ) have been measured, respectively, by a dc 4-probe technique and coulometric titrometry on the system of polycrystalline Ti0.99Ga0.01O1.995−δ against oxygen activity in the range of –20 < log aO2≤ 0 at different temperatures in the range of 1073 ≤T/K ≤ 1373. It is found that isothermal conductivity varies as 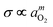 with m≈–1/4, –1/5, –1/4, +1/4, in order of increasing aO2, finally exhibiting an n-type (m = −1/4) to p-type (m = +1/4) transition crossing the stoichiometric composition (δ = 0), in perfect agreement with the oxygen nonstoichiometry variation. The electrical conductivity and oxygen nonstoichiometry isotherms are combined to evaluate the ionic partial conductivity and transference number, the intrinsic electronic excitation equilibrium constant, and eventually both mobilities of electrons and holes without employing any assumption at all. The partial molar enthalpy and entropy of the component oxygen are also evaluated as functions of nonstoichiometry.
with m≈–1/4, –1/5, –1/4, +1/4, in order of increasing aO2, finally exhibiting an n-type (m = −1/4) to p-type (m = +1/4) transition crossing the stoichiometric composition (δ = 0), in perfect agreement with the oxygen nonstoichiometry variation. The electrical conductivity and oxygen nonstoichiometry isotherms are combined to evaluate the ionic partial conductivity and transference number, the intrinsic electronic excitation equilibrium constant, and eventually both mobilities of electrons and holes without employing any assumption at all. The partial molar enthalpy and entropy of the component oxygen are also evaluated as functions of nonstoichiometry.


 Please wait while we load your content...
Please wait while we load your content...