A two-directional approach to the anatoxin alkaloids: second synthesis of homoanatoxin and efficient synthesis of anatoxin-a†‡
Abstract
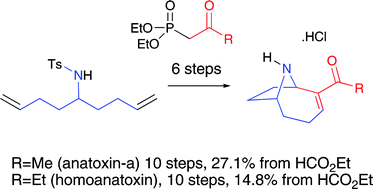
Total syntheses of the potent neurotoxins, environmental hazards, and biochemical probes anatoxin-a and homoanatoxin have been achieved from a common intermediate using a combined two-directional synthesis–tandem reaction strategy which includes key advances in oxidative desymmetrisation, tandem Michael–intramolecular Mannich cyclisations and a new method for deprotection of N-tosyl anatoxin-a.

 Please wait while we load your content...
Please wait while we load your content...