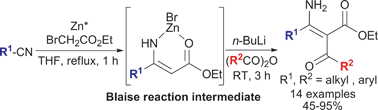
The first chemoselective tandem acylation of the Blaise reaction intermediate: a novel method for the synthesis of α-acyl-β-enamino esters, key intermediate for pyrazoles†
Abstract
The Blaise reaction intermediate, a zinc bromide complex of β-enamino ester, could be activated in situ by addition of a stoichiometric or catalytic amount of n-BuLi to allow chemoselective tandem C2-acylation providing α-acyl-β-enamino esters, which are valuable intermediates for the syntheses of tri- and tetrasubstituted

 Please wait while we load your content...
Please wait while we load your content...